By: Jay Lau
Throughout the fall, Harvard Chan faculty will share evidence-based recommendations on urgent public health issues facing the next U.S. administration. Ronnie Levin, instructor in the Department of Environmental Health, offered her thoughts on policies that could address contamination in the country’s drinking water supply.
Q: Why is drinking water quality a pressing public health issue?
A: The U.S. has arguably the best and most reliable drinking water in the world, and that’s because we’ve spent a lot of money and time getting it in that shape. On the other hand, our drinking water is not risk-free. It’s not perfectly safe—it can contain lead, nitrate, PFAS, arsenic, and uranium, as examples. In addition, there are racial and ethnic disparities in contaminant exposures, so not everybody gets the same quality of drinking water.
Q: What are the biggest challenges facing the next administration around improving drinking water quality?
A: A hundred years ago, we sunk a lot of money into water treatment and infrastructure, but then we stopped putting in that kind of investment. Now our water systems are severely aging and deteriorated, and we haven’t continued to maintain the older ones. And when we build new ones, they’re not always as well designed as the old ones.
In addition, science has moved on—we’ve found things in our drinking water that we thought weren’t bad, like PFAS, that turn out to be biologically active at very low levels. Lead, arsenic, and nitrate cause health effects at lower levels than we knew when we set the standards decades ago. We now need to catch up.
Ronnie Levin. Photo: Kent Dayton
Q: What are your top policy recommendations to address drinking water quality?
A: There’s been recent progress toward reducing lead in drinking water. On Oct. 8, the Environmental Protection Agency (EPA) announced a rule that requires all lead pipes in U.S. water systems to be replaced within the next decade, lowers the current level for taking action to reduce lead exposure from 15 to 10 parts per billion, and also implements several other policies to reduce exposure to lead from drinking water. If the rule is implemented and enforced, millions of people will have cleaner, safer water. Importantly, it will particularly enhance environmental protection among disadvantaged and low-income populations, which have been disproportionately impacted by lead-contaminated water.
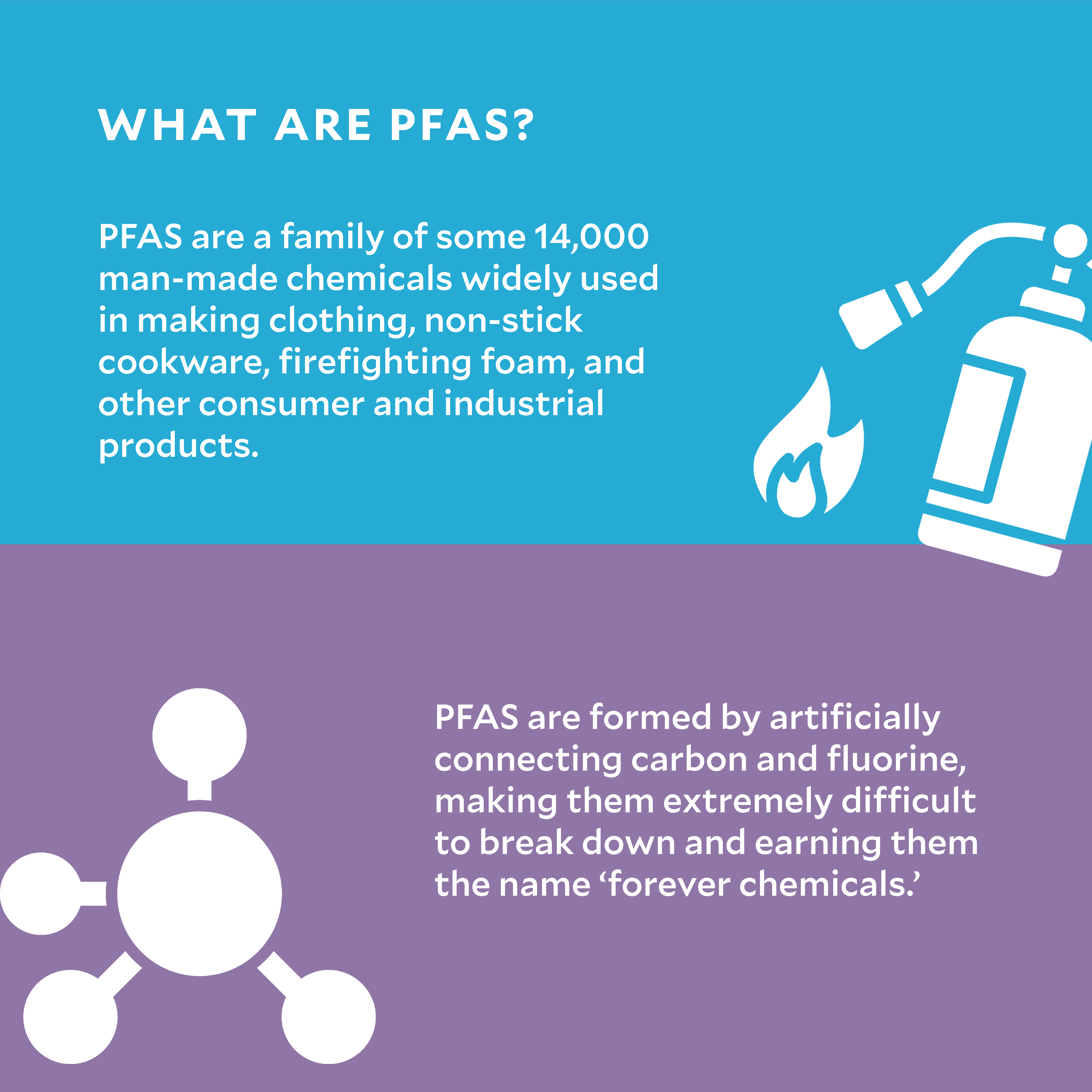
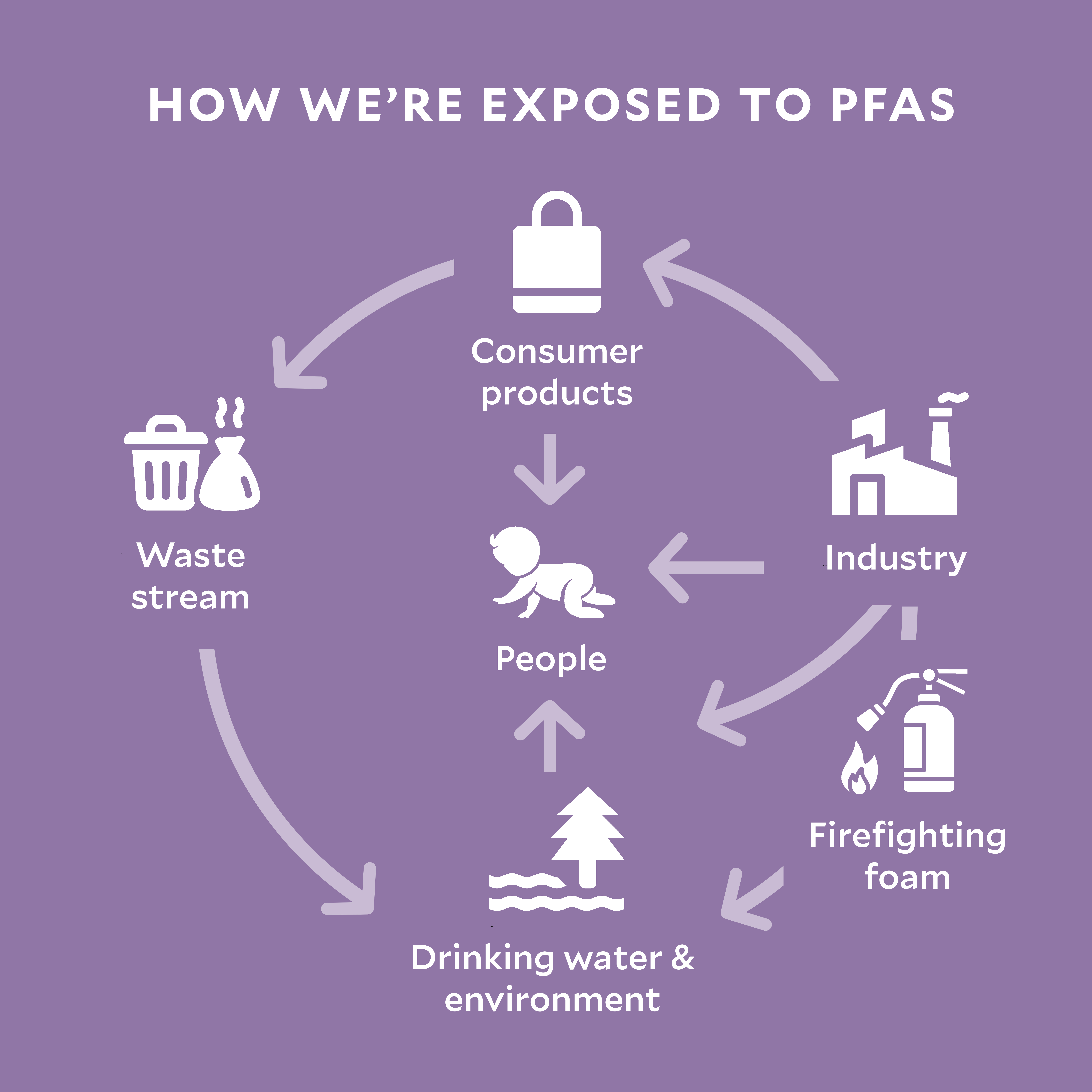
There are several other contaminants I’d like to see the government address. PFAS are a class of thousands of chemicals that are in all kinds of consumer products. We don’t even know all of them, because industry keeps tweaking them to be different and cheaper, and industry doesn’t have to report how they get used. There are PFAS everywhere, contaminating water, soil, air, and food, and they build up in people and the environment over time.
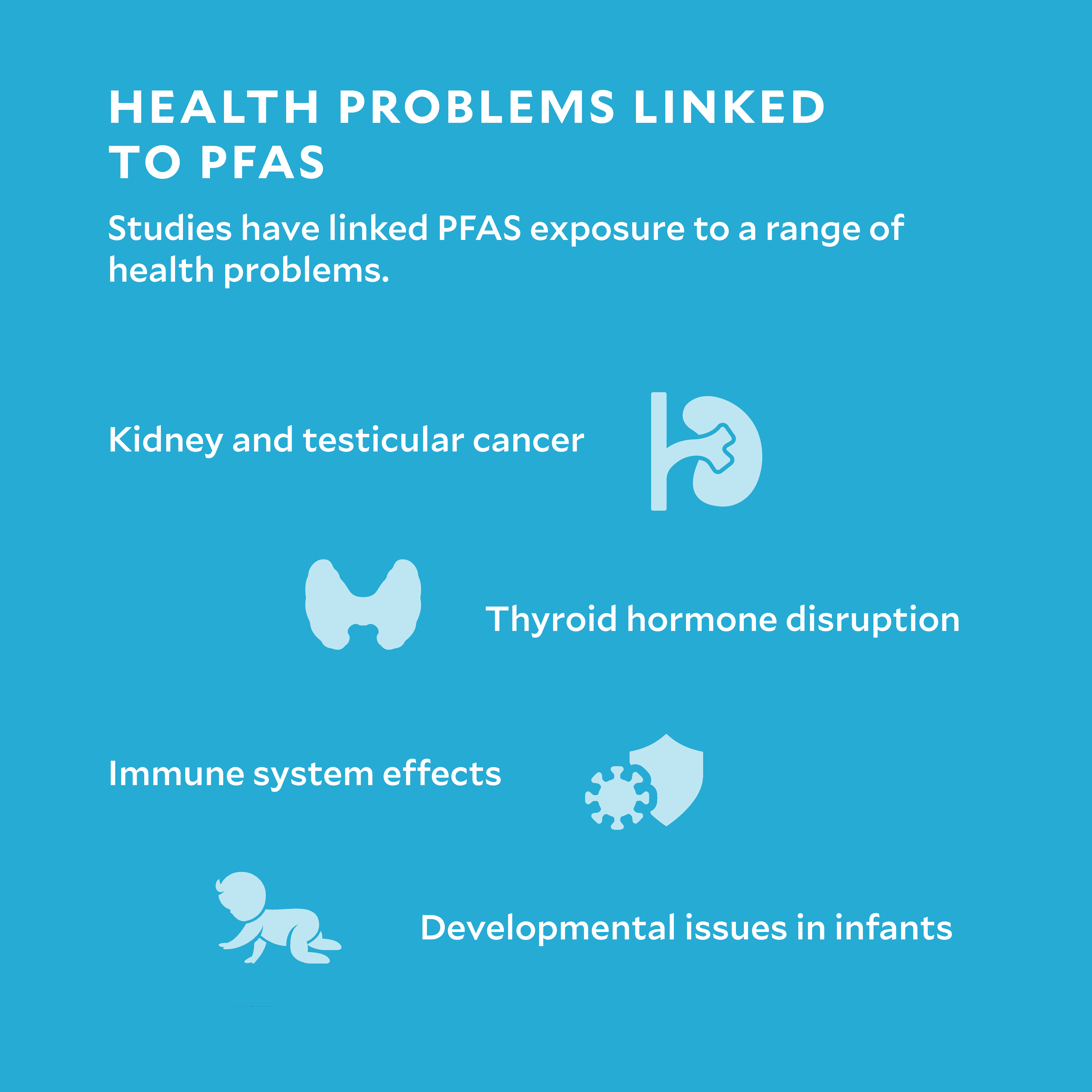
We don’t know a lot about the thousands of different PFAS because it takes years to do studies, and we haven’t known about them for that many years. But research so far has suggested that PFAS are associated with a host of biological changes, even at very low levels. PFAS exposure has been linked with many adverse health outcomes, such as decreased immune system function, thyroid disease, and kidney and testicular cancers.
The EPA recently set regulations for six PFAS chemicals, ones that we know are the easiest to measure and are associated with numerous health effects. Many people are researching PFAS, but industry is constantly altering the formulations for new and different applications, and so there’s no way to stop this train. But the EPA’s efforts are really good news.
Another issue that the EPA needs to address is revising the standard for arsenic in drinking water. We’ve known that arsenic is a poison for a really long time, and that’s actually what makes it so useful—we use it in chemotherapies, pesticides, and herbicides. It has a lot of other useful applications, like in paints and glassmaking. But arsenic has negative health effects across the board, including cardiovascular harms, liver damage, neurotoxicity, and reproductive toxicity.
The arsenic limit for drinking water—which was set in 2001 at 10 parts per billion—is probably an order of magnitude too high. It was looking like the EPA might propose lowering the arsenic standard in the next few years, but with the change in administration, that likelihood is looking dim. There’s a lot of resistance from industry and water utilities, but I think taking action on arsenic will be easier than regulating so many different types of PFAS, which is going to take a lot longer.
As for the EPA’s nitrate standard, it is dangerously high, and violations of the nitrate standard are the most common health-based violations of drinking water standards.
In general, the EPA is behind in keeping the drinking water standards up to date with the current scientific literature. Setting standards is a laborious process and, in addition, there is tremendous pushback from the “drinking water industry”—public water systems, which are often cities themselves, or semi-governmental agencies like the Massachusetts Water Resources Authority, which oversees water systems in the Boston metropolitan area. There are 50,000 active public water systems in the U.S., and there is a lot of complaining from those systems about the difficulty and expense of meeting stricter standards.
Q: What’s the evidence supporting those recommendations?
A: My colleagues and I wrote a 2023 review article about the exposure risks of a wide range of drinking water contaminants, including PFAS, arsenic, and more. In that article, we cited a number of studies linking these chemicals to health harms:
A 2023 meta-analysis of over a dozen different studies found that several types of PFAS may lower the body’s ability to produce antibodies after receiving vaccines, particularly for diphtheria, rubella, and tetanus.
A 2022 meta-analysis of over 100 rodent and human epidemiological studies identified a link between PFAS exposure and liver injury.
A 2013 study followed almost 4,000 individuals for around two decades, and found that higher arsenic levels in urine were associated with increased mortality from lung, prostate, and pancreatic cancers.
A 2015 meta-analysis of over a dozen studies identified a link between arsenic exposure and adverse pregnancy outcomes and infant mortality.
A 2021 study analyzed nationwide data collected by the Centers for Disease Control and Prevention, finding that an increased level of arsenic in urine was associated with heart disease mortality.
Q: What do you hope can be accomplished to improve drinking water quality in the next four years?
A: Addressing both PFAS and arsenic will be difficult and expensive, and also take a lot of political will. The Supreme Court has tied the EPA’s hands through decisions such as eliminating the Chevron deference, which called for deferring to federal agencies for their judgments where federal law is silent or unclear, and the 2023 Sackett v. EPA case, which limited the agency’s power to regulate wetlands and waterways. The EPA can’t just issue regulations, it has to get laws passed through Congress, which is much harder to do. It used to be that the courts would defer to the EPA, but now the agency is going to have to make a much stronger case for regulations.
Regarding lead, now that the Biden administration has finalized the new lead pipe rule the government needs to make sure that the rule is implemented and enforced.
We have to regain a commitment to protecting human health and the environment, and clean drinking water should be a top priority. We have a lot of hard work to do.
CLICK HERE FOR MORE INFORMATION